The fi gure below shows a “self-cooling” beverage can.
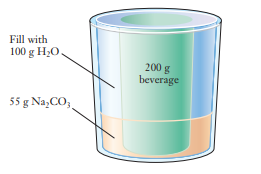
The can is equipped with an outer jacket containing sodium carbonate (Na2CO3), which dissolves in water rapidly and endothermically. Na2CO3(s) : 2 Na+ (aq) + CO3 2– (aq) DH ° = 67.7 kJ
The user adds water to the outer jacket, and the heat absorbed in the chemical reaction chills the drink. The can contains 200 g of drink, the jacket contains 55 g of Na2CO3, and 100 g of water is to be added. If the initial temperatures of the can and the water are 32°C on a summer day, what is the coldest temperature that the drink can reach? The can itself has a heat capacity of 40 J/°C.
Assume that the Na2CO3 solution and the drink both have the same heat capacity as pure water, 4.184 J g–1 °C–1 . (HINT: Treat this like a calorimetry problem.)