Inorganic Chemistry Slater’s Rules
Question :
1. Considering that:
Effective nuclear charge 2* = Z- S, where Z= nuclear charge and S = shielding constant And considering the Slater's rules (Attachment)
Calculate the Z* and S for Co'
2. Draw a qualitative molecular orbital diagram for the molecule of SiO
3. Determine the Lewis structure and geometry of SbC15
4. Determine the point group, symmetry operations of the molecule/ion PC1sand indicate the symmetry labels of each bond
Answer :
Solution 1: Slater’s rule: The general principle behind Slater's Rule is that the actual charge felt by an electron is equal to what you'd expect the charge to be from a certain number of protons, but minus a certain amount of charge from other electrons. Slater's rules allow you to estimate the effective nuclear charge Zeff. Zeff from the real number of protons in the nucleus and the effective shielding of electrons in each orbital "shell" (e.g., to compare the effective nuclear charge and shielding 3d and 4s in transition metals). Slater's rules are fairly simple and produce fairly accurate predictions of things like the electron configurations and ionization energies.
Electronic configuration of Cobalt (II) ion: (1s2) (2s2 2p6) (3s2 3p6) 3d7
The electron of interest is in d-orbital of cobalt ion and hence the shielding constant is evaluated as:
S=1×18+0.35 ×6=20.1
The nuclear charge of Cobalt is 27, hence the effective nuclear charge on cobalt ion is evaluated as:
Z^*= Z-S=27-20.1=6.9
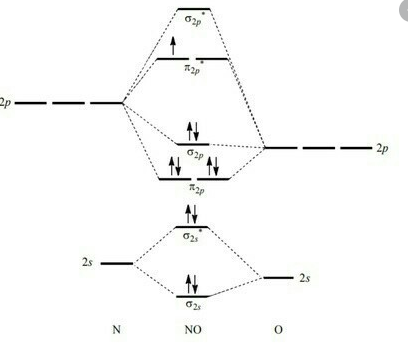
Solution 2: Qualitative Molecular Orbital diagram of SiO is shown below:
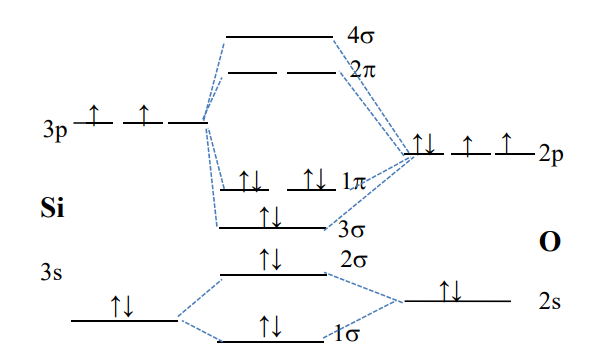
The 3p energy of Si is higher than that of O2p but the 3p-3s gap in Si is greater than 2p-2s gap in O. It all makes the order of AO and MO as shown in the sketch. The electron configuration for SiO than is 12 22 32 14.
Solution 3: Lewis structure of SbCl5 is shown below:
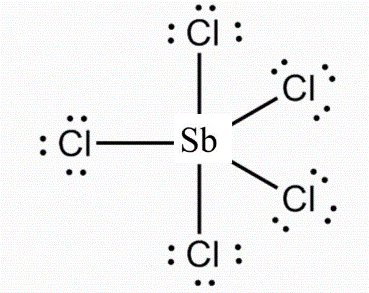
Geometry Details are as follows:
Molecular shape: Tridiagonal bipyramidal
Bond angle: 90° and 120°
Hybridisation of atom: Sb – sp3d and Cl-sp3
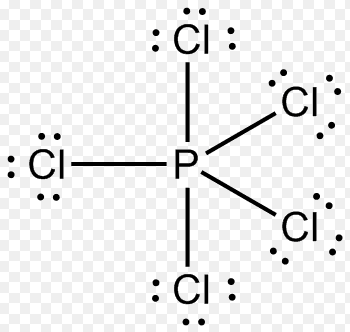
Solution 4: PCl5 contains a C3 main rotation axis and 3 perpendicular C2 axes. There are 3 σv planes and a σh plane. Hence PCl5 belongs to the D3h point group. P has 5 valence electrons plus 1 for each P-Cl single bond
Total = 10 electrons, five pairs
Structure - trigonal bipyramid, class - AX5, Point group- D3h
Solution 5: Bronsted acid: HClO4, Bronsted base: CH3CN, Lewis acid: HClO4, Lewis base: CH3CN. The equilibrium is in the backward direction.
Solution 6: Copper sulphate react with ammonia to produce pentaamminecopper(II) sulfate. The reaction proceeds at room temperature.
CuSO4 + 5NH3 → [Cu(NH3)5]SO4
Solution 7: SiO2 has a linear shape with the two oxygens connected to the Si by double bonds. There are no lone pairs on the Si. This would be an example of sp hybridization. SiO2 does not have a linear structure because it tends to form a bulky 3-D compound by bonding with the O atoms in such a way that it forms a chain.
Solution 8: Silicon dioxide has a huge variety of structures. Most of them have a tetrahedral SiO2SiOX2 unit cell — the O−Si−OO−Si−O angle is 109.5°109.5°, accordingly. The VB/hybridization approach to this tetrahedron would consequently assign 4 sp34 sp3 orbitals to the silicon atom.
Solution 9:
Solution 10:
Solution 16: In vapor-phase synthesis of nanoparticles, conditions are created where the vapor phase mixture is thermodynamically unstable relative to formation of the solid material to be prepared in nanoparticulate form. This includes usual situation of a supersaturated vapor. It also includes what we might call ‘chemical supersaturation’ in which it is thermodynamically favourable for the vapor phase molecules to react chemically to form a condensed phase. If the degree of supersaturation is enough, and the reaction condensation kinetics permit, particles will nucleate homogeneously. Once nucleation occurs, remaining supersaturation can be relieved by condensation or reaction of the vapor-phase molecules on the resulting particles, and particle growth will occur rather than further nucleation. Therefore, to prepare small particles, one wants to create a high degree of supersaturation, thereby inducing a high nucleation density, and then immediately quench the system, either by removing the source of supersaturation or slowing the kinetics, so that the particles do not grow. In most cases, this happens rapidly (milliseconds to seconds) in a relatively uncontrolled fashion and lends itself to continuous or quasi continuous operation. This contrasts with many colloidal syntheses of nanoparticles that are carried out in discrete batches under well-controlled conditions with batch times of hours to days.
Chemical Process Synthesis: In this approach, vapor phase precursors are brought into a hot-wall reactor under conditions that favour nucleation of particles in the vapor phase rather than deposition of a film on the wall. It is called chemical vapor synthesis or chemical vapor condensation in analogy to the chemical vapor deposition (CVD) processes used to deposit thin solid films on surfaces. This method has tremendous flexibility in producing a wide range of materials and can take advantage of the huge database of precursor chemistries that have been developed for CVD processes. The precursors can be solid, liquid or gas at ambient conditions, but are delivered to the reactor as a vapor (from a bubbler or sublimation source, as necessary).
Solution 17: Single Walled Carbon Nanotubes are defined as a one dimensional, cylindrically shaped allotropes of carbon that have a high surface area and aspect ratio (length to diameter ratio). Single-walled carbon nanotubes (SWCNTs) possessing outstanding linear and nonlinear optical properties are utilized as saturable absorbers (SAs) for passive mode-locking of different bulk solid-state lasers in a wide spectral range between 0.8 and 2 μm. To overcome some of the limitations and drawbacks of currently available saturable absorbers, diverse carbon nanotubes and techniques for fabrication of single-walled carbon nanotube saturable absorbers (SWCNT-SAs) are investigated. In this chapter, we discuss various manufacturing procedures and essential characteristics of SWCNT-based passive mode-locking devices and review recent demonstrations of passively mode-locked solid-state lasers employing different types of SWCNT-SAs.
Solution 20: Geometry of [Fe (CO)5]
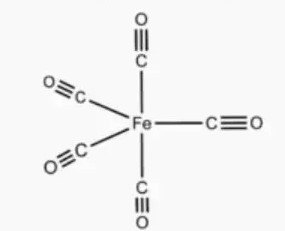
It's called “synergic bonding”. The ligand (CO) donates its lone pair of electrons to the vacant orbitals of the iron atom and forms the sigma-bond. Since the iron atom also possesses some electrons in its d-orbitals, it back donates those electrons to the molecular orbitals of the ligand forming a π-bond. In this way the metal-carbon bond length is reduced, and the complex gets more stability. One important thing to keep in mind is that the metal atom donates it's electron pairs to the antibonding MO of CO , so the C-O bond is weakened by this synergic bonding , leading to a larger C-O bond length in the complex (as opposed to a free CO molecule).













