5 Star Rating
Orders Deliver
PhD Experts
Support
Privacy
Top Quality
Number Of View : 70
Download : 0
Pages: 5
Words : 1131
Question 1
For the element Arsenic a) Write the electronic configuration and localization in the periodic table b) Draw the molecular orbital of the molecule As2 c) Indicate (if it is possible) in which cases this element can form ionic bonds and when they can form covalent bond d) Explain which type of bonding interaction this element As can have with hydrogen, and mention 5 compounds they could form e) Explain which type of bonding interaction this element As can have with oxygen, and mention 5 compounds they could form f) Compare the physical and chemical properties of this element As with the others in the same group g) Which are the oxidation states of this element As, give three examples of compounds in which the element has each of the oxidation states indicated here. h) Explain why this element, As, and/or its compounds are important.
Question 2
a) Write the formula of the complexes that could be formed by Rhenium and the ligand Cl and Rhenium and the ligand ethylenediamine, and write their names. b) Draw the structure of these Rhenium complexes c) Indicate all the isomers (how many and specify the type of isomers) of each compound and give their names
Question 3
a) For the complexes in the former question indicate the magnetic susceptibility b) For the complex formed by Cobalt(II) and 4 Cl ligands, indicate and draw the crystal field splitting locating the electrons and calculate the ligand field stabilization energy. Explain if it is possible to form pi bonds and if these could be stable c) For the complex formed by Cobalt and 6 (NH3) ligands indicate and draw the crystal field splitting locating the electrons and calculate the ligand field stabilization energy. Explain if it is possible to form pi bonds and if these could be stable
QUESTION 1
For the element Arsenic
a) Electronic configuration and localization in perodic table
Arsenic atoms have 33 electrons and the ground state electronic configuration of neutral arsenic is
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3
In the periodic table, Arsenic is present in the 15th column (group VA) and is placed at the third position; Nitrogen and Phosphorous occupying 1st and 2nd position at the top of the column. Having some properties resembling to metals and others to non-metals, it has been classified as a metalloid. It is similar in oxidation state and electron orbital status with phosphorus.
b) Molecular orbital of the molecule AS2
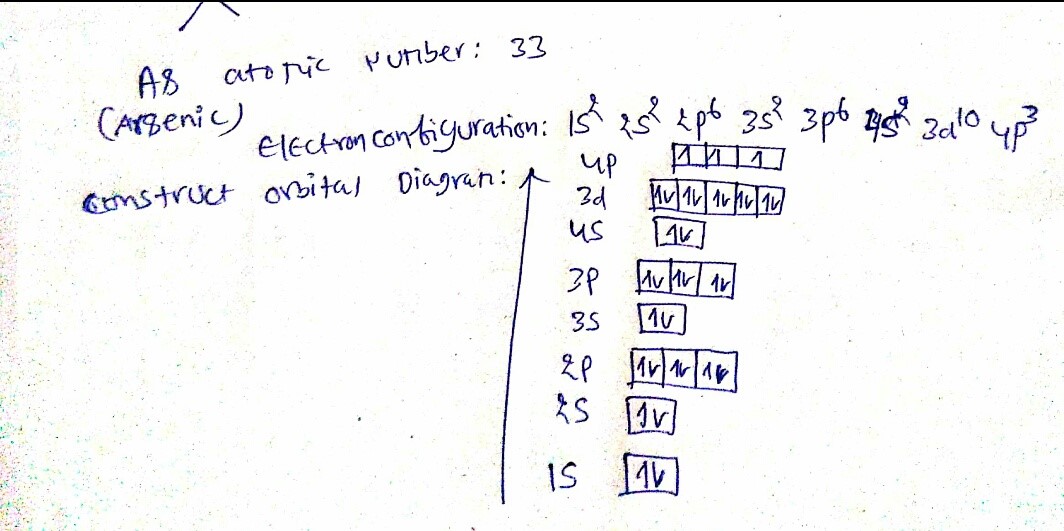
Figure 1: Orbital Configuration of Arsenic Molecule
c) The type of bond formed by Arsenic is affected by its electronic configuration. It has the ability to readily change oxidation state and bonding configuration. This behavior is a consequence of the electronic configuration of its valence orbitals, with partially filled states capable of both electron donation and overlap in covalent bonds. For example, A large number of metals arsenides are known and can be considered as alloys of metals, generally with the form MeAsn, where n = 1, 2, or 3 and Me is a metallic element. Electron counting would indicate a negative formal oxidation state for arsenic in these compounds. However, bonding is strongly covalent in these semiconducting solids.
d) Arsenic forms trihydride (AsH3), one of the simplest arsenic compounds, the highly toxic flammable gas, with hydrogen. The other compounds of arsenic containing hydrogen are Arsenic Acid, Monomethylarsonic acid (MMA), Dimethylarsinic acid (DMA).
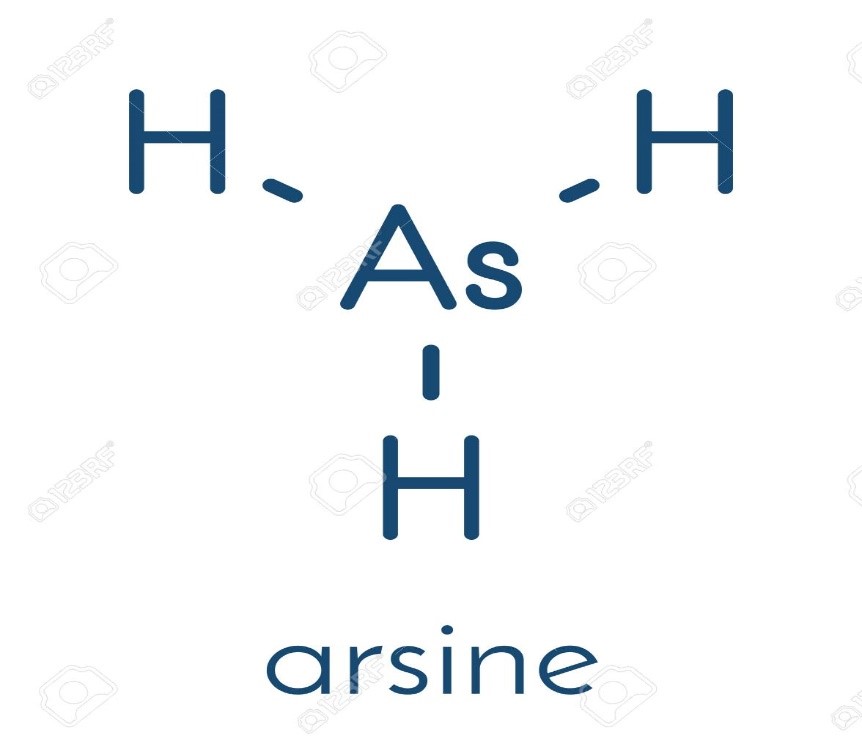
Figure 2: Trihydride of Arsenic with Hydrogen
e) Arsenic reacts with oxygen to form arsenic oxide. The possible compounds of arsenic with oxygen could be arsenic trioxide (As4O6), AsO3, Arsenic pentaoxide can not be prepared by direct reaction of arsenic with oxygen. It is prepared by oxidation of arsenic with nitric acid. Other compounds of arsenic containing oxygen are, As2O3.
f) Nitrogen and Phosphorus represent the two most valuable elements of the group VA with Arsenic, Antimony and Bismuth the other elements. They differ very much in their physical and chemical properties. Nitrogen is a gas and nonmetallic. Its diatomic molecule has three bonds. Phosphorus is also a non-metallic element and in vapor form it is found as tetrahedral P4 molecules and in solid form it exists as the white allotropic form. Arsenic is metalloid and exists as solid at room temperature. Antimony is a semimetal and has two allotropes. Like Arsenic, the metallic allotrope of Antimony is more stable.
Chemically nitrogen is inert, odorless, colorless and tasteless gas. It combines relatively with few other elements. The most common available form of phosphorous is white phosphorous which reacts readily with oxygen and catches fire. Phosphorous also reacts easily with halogens.
g) Two possible oxidation states of arsenic are 3 and 5. They are Arsenite, AsIII, and arsenate, AsV. The examples of the compounds are:
Arsenite: arsenic trioxide, sodium arsenite and arsenic trichloride, Zn3(AsO3)2, Pb5Cl (AsO3)3, Pb2As2O5
Arsenate: copper arsenate, lead arsenate and calcium arsenate
h) The compounds of Arsenic and Arsenic itself can be utilized in various range of uses both industrial and domestic. They had been used in applications such as pharmaceuticals, agricultural chemicals, pesticides, and wood preservatives. Arsenic has also found its applications in semiconductor, mining, glass making and metallurgical factories. Historically, the agricultural industry had been employing Arsenic and its compounds as insecticides, pesticides and herbicides. Arsenic is also used in the formation of alloys of lead. Because Arsenic is a common n-type dopant, so due to this property it is widely used in semiconductor electronic devices. Gallium arsenide stands at 2nd position after doped silicon as most commonly used semiconductor.
Question 2
a) Rhenium complexes with cholorine : Rhenium trichloride (ReCl3), Rhenium pentachloride (ReCl5), Rhenium Hexachloride (Recl6).
b) Structures of the complexes
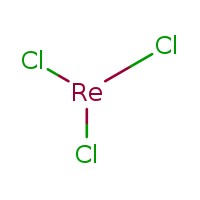
Figure 3: Rhenium Trichloride
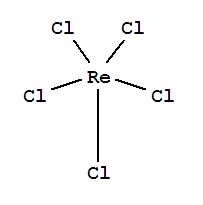
Figure 4: Rhenium Pentachloride
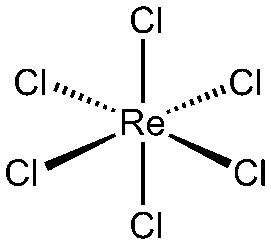
Figure 5: Rhenium Hexachloride
Question No. 3
a) Magnetic susceptibility
Under the application of the magnetic field, the ability of a material to become magnetized is measured through susceptibility. Mathematically, it is represented by the ratio of magnetization M (magnetic moment per unit volume) to the applied magnetizing field intensity H. The most materials can be classified into 2 simple classes based on their response to an applied magnetic field.
Those aligned with the magnetic field, χ > 0, called paramagnetism, and others having an alignment against the field, χ < 0, called diamagnetism.
b) Crytal field splitting for [CoCl4]2- is shown in below figure:
This complex has a coordination number of 4, so it can have tetrahedral or square planar geometry. Tetrahedral complexes are favored where attainment of regular complexes is important. For the tetrahedral complexes d0,d2,d5,d7d0,d2,d5,d7, and d10d10 the configurations are regular.
In tetrahedral complexes, mostly Δt
Co2+ ions = d7 configuration
t2 has 3 electrons while e has 4 electrons.
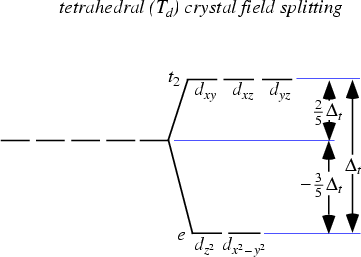
Calculation of field stabilization energy
Higher orbitals have energy value of +0.4Δt and the lower orbitals have the energy value of −0.6Δt.
CFSE=+0.4(3) −0.6(4) =−1.2
c) Crytal field splitting for [Co (NH3)6]3+ is shown in below figure:
Co3+ ions = d6 configuration
t2 has 4 electrons while e also has 4 electrons.
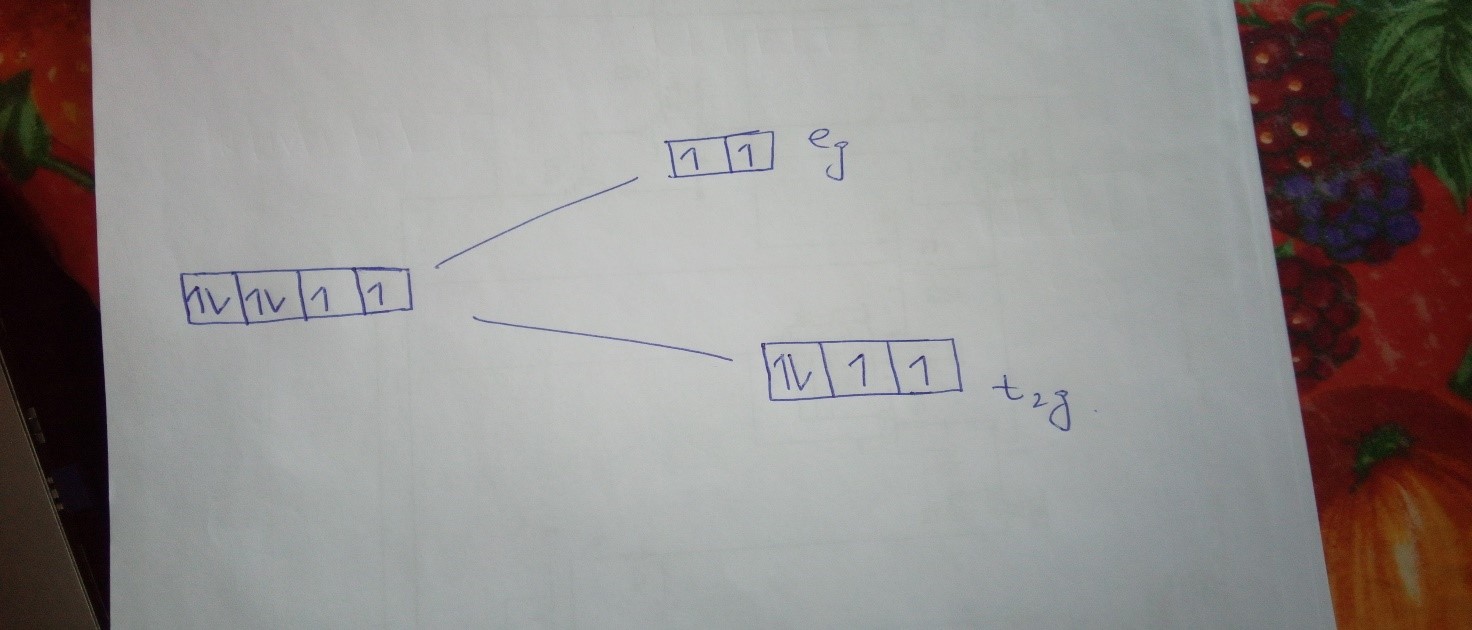 Figure 6: Crystal field splitting for Hexaammonium Cobalt
Figure 6: Crystal field splitting for Hexaammonium Cobalt
CFSE=+0.4(2) −0.6(4) =−1.6
Limitless Amendments
$09.50 free
Bibliography
$10.50 free
Outline
$05.00 free
Title page
$07.50 free
Formatting
$07.50 free
Plagiarism Report
$10.00 free
Get all these features for $50.00
Enter your email, and we shall get back to you in an hour.